HIV-HCV-HBV-RPP30RNA2
Quantity: 100 x 20μL PCR reactions
Detects: HIV type I, Hepatitis C virus, Hepatitis B virus, and Human RPP30 mRNA
(RUO). Research Use Only. Not for use in Diagnostic Procedures.
Cat #: HIV-HCV-HBV-RPP30RNA2-TC-0045
Introduction
The assay HIV-HCV-HBV-RPP30RNA2 is a multiplexed realtime reverse transcription polymerase chain reaction (RTqPCR) test intended for the qualitative detection of nucleic acid from human immunodeficiency virus type 1 (HIV-1), human hepatitis C virus (HCV), and human hepatitis virus B (HBV). The HIV assay detects the gene for “pol protein” (Protein ID: QJQ91623.1). The HCV assay detects the gene for “polyprotein” (Protein ID: ACJ04208.1). The HBV assay detects the gene for S protein (Protein ID: QJC70195.1). This kit is for research use only and should not be used for diagnostic procedures. The multiplexed qPCR reaction can simultaneously detect HIV, HCV, HBV, and the control human RPP30 spliced mRNA with detection at emission wavelengths of: 610 nm (CalFluorRed610™), 517 nm (FAM™), 670 nm (Cy5™), and 554 nm (HEX™), respectively.1 The assay requires the use of a qPCR instrument with multiple detection channels and was validated on a Bio-Rad CFX96™ Real-Time System, but other qPCR instruments may also be suitable for this assay.

Tube 1: 20X primer/probe mix to detect HIV-1, HCV, HBV, and spliced human RPP30 mRNA.
Contents
Tube 1 is provided as a 20X concentrated working solution that contains a mixture of all the primers and probes needed for the multiplexed detection of HIV, HCV, HBV, and the control human RPP30 spliced mRNA. Performing the reaction requires RT-qPCR mastermix: highQu One-Step RT-qPCR Probe Kit from highQu GmBH (item: QOP0405, provided separately from DNA Software or you can order directly from highQu GmbH).
Note: molecular biology grade water should be used to prepare the PCR reactions, which is NOT included in this kit.
Kit Handling and Contamination
The DNA Software assay HIV-HCV-HBV-RPP30RNA2 is shipped at ambient temperature and should be stored at -30 to -15°C. The kit should be kept on ice once thawed.
Any contamination should be avoided by using appropriate personal protective equipment (PPE), powder free gloves, aerosol barrier pipette tips, and a clean hood.
Protocol
Thaw contents on ice. Mix well before use. Prepare your 20 μL
reaction as follows:
| Component | Volume (µL) |
|---|---|
| highQu qPCR mastermix (4X) | 5 |
| highQu RT enzyme (20X) | 1 |
| Primer/probe mix (20X) | 1 |
| Sample | 2 |
| Water | 11 |
Note: The composition of this reaction is calculated based on the user manual of highQu One-Step RT-qPCR Probe Kit from highQu GmBH (items: QOP0405). The volume of water should be adjusted accordingly if the user’s reaction preparation is different from the recommended preparation method.
A RT-qPCR protocol was used at DNA Software, Inc. for prevalidation on a Bio-Rad CFX96™ Real-Time System, with the following program:
| Step | Thermocycling Protocol: |
|---|---|
| 1 | Incubate @ 50 °C for 5 minutes |
| 2 | Incubate @ 95 °C for 3 minutes |
| 3 | Incubate @ 95 °C for 15 seconds |
| 4 | Incubate @ 58 °C for 30 seconds |
| 5 | Plate Read |
| 6 | Go to Step 3, repeat 44x more |
| 7 | (optional) Incubate @58 °C for 3 minutes |
Result Interpretation
After running the RT-qPCR reaction, perform a regression analysis on the data to determine the quantification cycle, Cq (Cq is preferred over Ct). Each fluorescence channel with a Cq < 38 cycles and final RFU >200 is considered “positive” or “+” in the Table below.
| HIV (CalFluorRed610™) |
HBV (Cy5TM) |
HCV (FAM™) |
RPP30 (HEX™) |
Interpretation and recommendation |
|---|---|---|---|---|
| – | – | – | – | The PCR reaction failed. Please repeat the experiment |
| – | – | – | + | The sample doesn’t contain HIV, HCV, or HBV. |
| + | – | – | – | The sample contains HIV RNA. The sample does not contain HBV or HCV. The sample may not contain spliced human RPP30 mRNA. |
| + | – | – | + | The sample contains HIV RNA and spliced human RPP30 mRNA. The sample does not contain HBV or HCV. |
| – | + | – | – | The sample contains HBV DNA. The sample doesn’t contain HIV or HCV RNA. The sample may not contain spliced human RPP30 mRNA. |
| – | + | – | + | The sample contains HBV DNA and spliced human RPP30 mRNA. The sample does not contain HIV or HCV RNA. |
| – | – | + | – | The sample contains HCV RNA. The sample does not contain HIV or HBV. The sample may not contain spliced human RPP30 mRNA. |
| – | – | + | + | The sample contains HCV RNA and spliced human RPP30 mRNA. The sample does not contain HIV or HBV. |
| + | + | – | – | The sample contains both HIV and HBV. The sample does not contain HCV RNA. The sample may not contain spliced human RPP30 mRNA. |
| + | + | – | + | The sample contains HIV, HBV, and spliced human RPP30 mRNA. The sample does not contain HCV RNA. |
| + | – | + | – | The sample contains both HIV and HCV RNA. The sample does not contain HBV DNA. The sample may not contain spliced human RPP30 mRNA. |
| + | – | + | + | The sample contains HIV, HCV RNA and spliced human RPP30 mRNA. The sample does not contain HBV DNA. |
| – | + | + | – | The sample contains HBV and HCV. The sample does not contain HIV RNA. The sample may not contain spliced human RPP30 mRNA. |
| – | + | + | + | The sample contains HBV, HCV, and spliced human RPP30 mRNA. The sample does not contain HIV RNA. |
| + | + | + | – | The sample contains HIV, HBV, and HCV. The sample may not contain spliced human RPP30 mRNA. |
| + | + | + | + | The sample contains HIV, HBV, HCV and spliced human RPP30 mRNA. |
Pre-Validation Data
The assay pre-validation was carried out as quadruplex reaction, HIV-HCV-HBV-RPP30RNA2, where HIV is detected in the CalFluorRED610 channel, HCV in the FAM channel, and HBV in the Cy5 channel. Human RPP30 mRNA is detected in
the HEX channel and serves as a positive control.
Experiments were performed in triplicate using the experimental procedure given above. The samples used for the validation experiments include HIV, HCV, and HBV 500 BP long synthetic DNA constructs (from Twist Biosciences) harboring the regions of interest from the three viral genomes, and the human total brain RNA (Takata Cat#636530). Each of the standard samples or each of the viral DNA constructs in combination with human total brain RNA was added. The results of these experiments are shown in Figure 1. Figure 2 shows serial dilution experiments that indicate a limit of detection (LOD) <10 molecules for the 500 BP synthetic DNA constructs of the three targets. RNA samples of HIV (NATHIV1-LIN, BSL-1) and HCV (NATHCV-0005, BSL-1) from ZeptoMetrix were employed to test the performance of the multiplex assay using extracted pathogen RNA samples. The results of these experiments are shown in Figure 3.

Figure 1: Pre-validation experiments with single target or a target in combination with the human total brain RNA (given in text boxes). The full set of 8 primers and 4 probes are included in every reaction, but positive signal is only observed when the target is present, indicating that the amplification is specific, and the performance of the assay is not affected by the positive control sample.
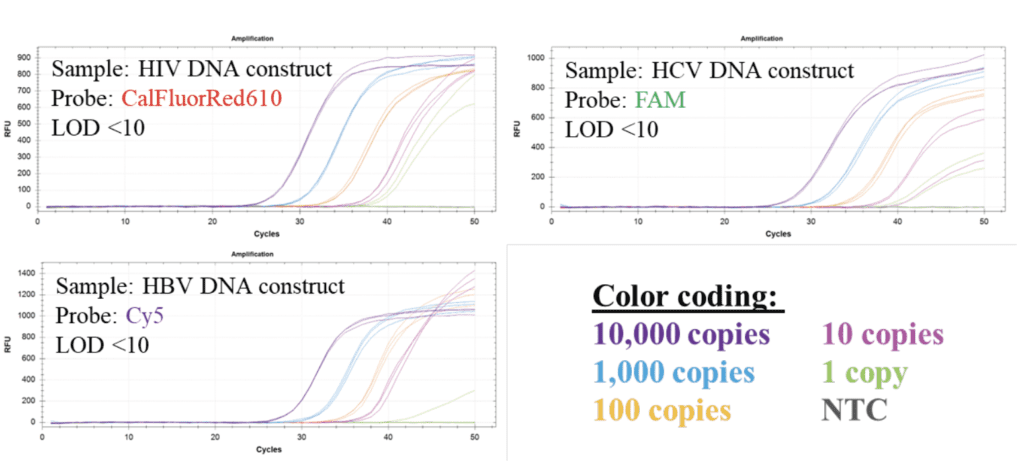
Figure 2: Serial dilution experiments show LOD <10 molecules for all DNA constructs of the three viral genomes. The full set of 8 primers and 4 probes are included in every reaction. All NTC reactions show no signal.
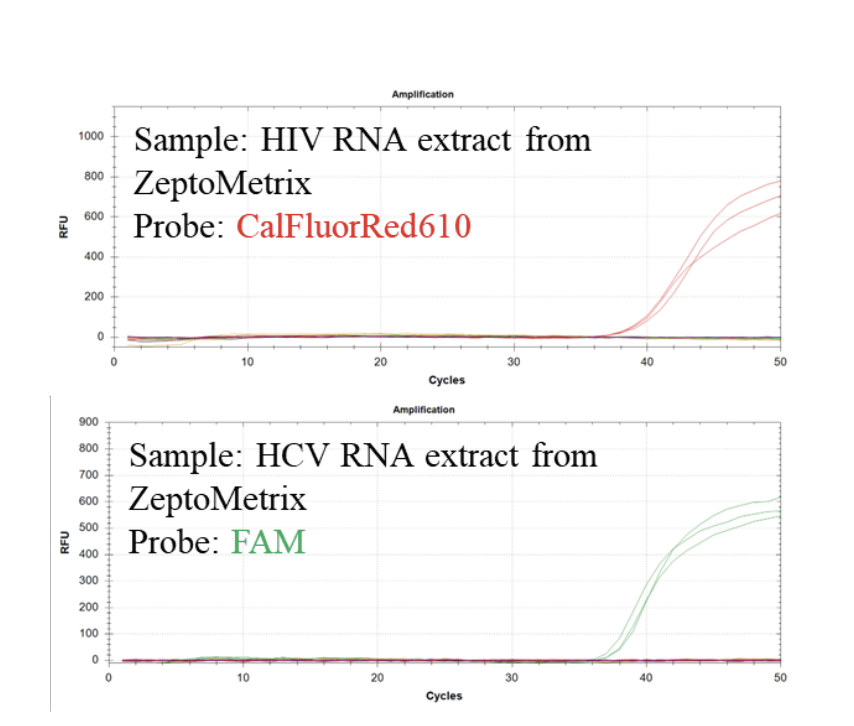
Figure 3: Multiplexed qPCR detection of extracted pathogen RNA from HIV and HCV (obtained from Zeptometrix, Inc.). Positive signals were only observed when the corresponding RNA extract was added.
Conclusion: The data in Figure 1 indicate that the primers and probes of the HIV, HCV, HBV, and the RPP30RNA control assays are compatible with each other and detect the targeted pathogen gene with specificity.
Limit of detection (LOD) was estimated by performing serial dilution experiments in triplicate (Figure 2). For dilution series only one of the DNA constructs of the three viral targets was added. The results show a limit of detection (LOD) <10 copies/reaction.
This multiplex assay can detect HIV and HCV in samples containing extracted viral RNA (Figure 3).
Contact Us
For assistance, please contact DNA Software using the link: https://www.dnasoftware.com/contact/
Address:
Michigan Life Science and Innovation Center
46701 Commerce Center Dr
Plymouth, MI 48170
Phone: (734) 222-9080
Notes:
1 Note that CalFluorRed610™, FAM™, Cy5™, and HEX™ are trademarks of Biosearch Technologies, Inc, Life Technologies, Inc, GE Healthcare, and Applera Corp, respectively.

